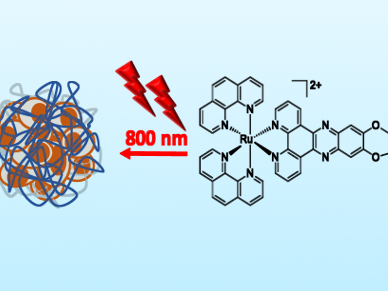
Photodynamic therapy (PDT) has become a viable treatment against certain types of cancers. PDT uses molecules, e.g., metal complexes, that can generate reactive oxygen species within cancer tissues upon light-activation. The advantage of using metal complexes is the tunability of the ligands, which allows for the possibility of two-photon excitation (resulting in a lower energy of excitation and deeper tissue penetration). In addition, biologically active ligands can be used (e.g., as DNA intercalators).
Hui Chao, Sun Yat-Sen University, Guangzhou, China, Gilles Gasser, Chimie Paris Tech, PSL Research University, France, and colleagues have synthesized two [Ru(phen)2(dppz)]2+ complexes (phen = phenanthroline; dppz = dipyrido[3,2-a:2′,3′-c]-phenazine), differing only in the functional groups of the dppz ligand (Complex 1 has dppz-7,8-(OMe)2, and 2 has dppz-7,8-(OH)2, see scheme).
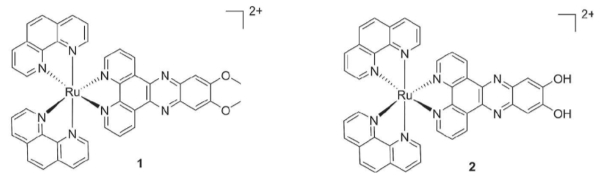
After characterizing the complexes using crystallographic and spectroscopic techniques, the team evaluated the toxicity of these complexes on a monolayer of HeLa cells. They showed that complex 1, while having some dark toxicity, had an IC50 = 3.1 μM upon one-photon activation (IC50 is a measure for the effectiveness of inhibition of a biological process). With this value, the complex outperforms the known chemotherapeutic drug cisplatin (IC50 = 29.5 μM) and the clinically approved PDT drug ALA (IC50 = 154.8 μM). Complex 1 also showed activity against 3D HeLa spheroids (representing solid tumors) when activated using two-photon excitation (IC50 = 9.5 μM, compared with IC50 = 32.5 μM with one-photon activation, 69.5 μM for cisplatin, and >200 μM for ALA).
- Evaluation of the Medicinal Potential of Two Ruthenium(II) Polypyridine Complexes as One- and Two-Photon Photodynamic Therapy Photosensitizers,
Jeannine Hess, Huaiyi Huang, Adrian Kaiser, Vanessa Pierroz, Olivier Blacque, Hui Chao, Gilles Gasser,
Chem. Eur. J. 2017, 23, 9888–9896.
DOI: 10.1002/chem.201701392