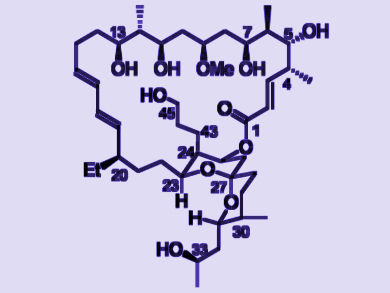
Finding the correct configurations of the antifungal marine macrolides neomaclafungins by NMR spectroscopy has been a challenge. This is due to a lack of observable 1H–1H coupling (also called nuclear Overhauser effect, NOE) between the two major moieties. Moreover, assignment by synthesis has been hampered by the absence of a clear-cut target structure.
Shijun Zhu and Yikang Wu, Shanghai Institute of Organic Chemistry, Chinese Academy of Sciences, have completed the first synthesis of neomaclafungin A (pictured). Their synthesis allowed the assignment of relative and absolute configurations of neomaclafungins. It also revealed that the configuration of one moiety relative to that of another remotely separated one may result in huge differences in reactivity, a phenomenon that was unexpected.
The team’s work also discloses some interesting abnormal regioselectivity at several steps and an unprecedented two-stage hydrolysis of acetonides, which may help when dealing with similar highly sensitive substrates.
- Synthesis and Configuration of Neomaclafungin A,
Shijun Zhu, Yikang Wu,
Chem. Asian J. 2017.
DOI: 10.1002/asia.201700950