Enantioselectivity is difficult to implement into chemical synthesis. In order to preferentially obtain one enantiomer, asymmetric catalysts or additives can be used. Otherwise, a racemic mixture is produced, which can often require expensive techniques in order to separate the enantiomers.
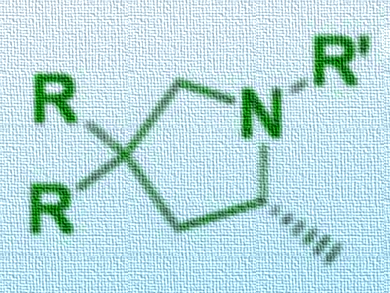
Francine Agbossou‐Niedercorn, Christophe Michon, Université de Lille, France, and colleagues have developed a method of controlling the enantioselectivity of a gold-catalyzed hydroamination reaction by simply changing the solvent. Using alkenes as substrates, cyclic amines (pictured) could be produced using a gold(I) salt and phosphine ligands as a catalyst and silver perchlorate as an additive. The team found that using methanol as a solvent yielded the R enantiomer in high yields with up to 58 % ee, whereas using toluene as a solvent gave the S enantiomer in up to 68 % ee.
It was determined using X-ray fluorescence spectroscopy that methanol, a polar solvent, allows for the formation of gold–silver adducts. This enables the formation of a dinuclear gold complex, in which one gold is coordinated with silver. This species has a large bite angle, enabling one gold atom to facilitate proton transfer, while the other is anchored to the other side of the substrate. This is cannot happen in toluene, and the proton transfer can only take place on the same face as the alkene coordination. This effect leads to opposite enantiomers being formed in the two solvents.
- Gold(I)-Catalysed Asymmetric Hydroamination of Alkenes: A Silver- and Solvent-Dependent Enantiodivergent Reaction,
Marc-Antoine Abadie, Xavier Trivelli, Florian Medina, Nathalie Duhal, Mostafa Kouach, Bernhard Linden, Eric Génin, Maxence Vandewalle, Frédéric Capet, Pascal Roussel, Iker Del Rosal, Laurent Maron, Francine Agbossou-Niedercorn, Christophe Michon,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201701301