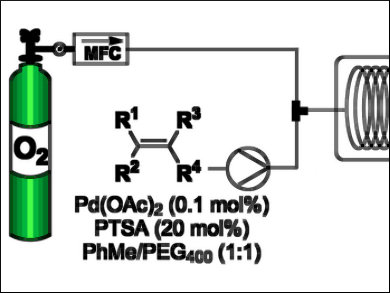
Molecular oxygen is an ideal terminal oxidant, but it is an underutilized reagent in organic synthesis, particularly at a large scale, because of its potential flammability and explosive properties under certain conditions. However, the properties of continuous-flow reactors enable the safe handling of pure O2 in many cases.
C. Oliver Kappe, University of Graz, Austria, and colleagues have developed a scalable procedure for olefin cleavage to form aldehydes and ketones, using pure O2 as the sole oxidant within a flow reactor. The reaction proceeds with a low catalyst loading of 0.1 mol% Pd(OAc)2 in the presence of PTSA (p-toluenesulfonic acid monohydrate) as an acid additive. The intensified flow process occurs at 120 ºC and 10 bar O2 pressure. Reaction times are about 25 minutes long, which is much faster than previously reported batch processes.
The team used poly(ethylene glycol)-400 (PEG-400) as a polymer co-solvent to stabilize the active palladium catalyst and prevent decomposition. The developed method can be expanded for the safe preparation of many valuable organic compounds using environmentally benign and inexpensive O2.
- A Continuous-Flow Process for Palladium-Catalyzed Olefin Cleavage by using Oxygen within the Explosive Regime,
Christopher A. Hone, Anne O’Kearney-McMullan, Rachel Munday, C. Oliver Kappe,
ChemCatChem 2017.
DOI: 10.1002/cctc.201700671