The synthetic fixation of the greenhouse gas carbon dioxide into fuel precursors and commodity chemicals is an important research challenge. When mediated by transition metal complexes, the so-called reductive disproportionation of CO2 into carbon monoxide and carbonate occurs rapidly via catalytic intermediates that are thought to contain metal-carbon multiple bonds.
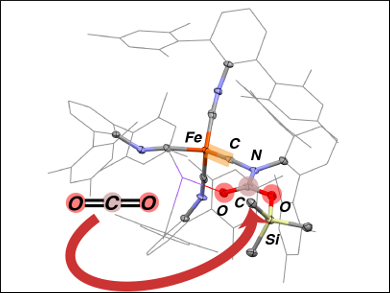
Using sterically encumbered ligands, Joshua S. Figueroa, University of California, San Diego, La Jolla, USA, and colleagues have trapped iron intermediates formed during CO2 reductive disproportionation. The transformation of CO2 took place when a dianionic iron complex bearing four bulky isocyanide ligands (namely tetraisocyanide dianion Na2[Fe(CNArMeS2)4] ) was used. However, when the complex and CO2 reacted in the presence of silyl triflates, the process was arrested by silylative esterification of a mono-CO2 adduct.
An electronic rearrangement of this adduct produced an iron terminal carbyne complex, which was isolated (pictured).In this unique trigonal-monopyramidal terminal carbynes, the Fe≡C bond is conformationally locked within the equatorial plane. Compounds featuring iron-carbon triple bonds are rare. They may yield interesting chemistry that can be further exploited for synthetic CO2 fixation.
- Terminal Iron Carbyne Complexes Derived from Arrested CO2 Reductive Disproportionation,
Charles C. Mokhtarzadeh, Curtis E. Moore, Arnold L. Rheingold, Joshua S. Figueroa,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201705877