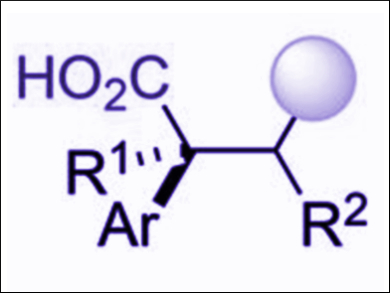
The use of CO2 as a reactant in organic transformations is a popular research target for chemists who want to incorporate this abundant chemical into useful molecules. Readily available styrenes, for example, can be converted into valuable phenylacetic acids by carboxylation with CO2. However, these reactions are usually carried out using air-sensitive organometallic reagents or stoichiometric metal reductants.
Ruben Martin, Institute of Chemical Research of Catalonia (ICIQ), Tarragona, Spain, and colleagues have developed a method for the difunctionalization of styrenes with carbon-centered radicals and CO2 (1 atm) under visible light irradiation in the presence of the iridium photocatalyst [Ir(ppy)2(dtbbpy)]PF6 (ppy = 2-phenylpyridine, dtbbpy = 4,4′-bis(tert-butyl)-2,2′-bipyridine). This reaction does not require sensitive reagents or metal reductants and tolerates a wide variety of sensitive functional groups, including esters, boronic acids, sulfonates, or amides.
The reaction is the first example of a redox-neutral carboxylation of styrenes in which no stoichiometric metal reductants or organometallic species are required, and has a complementary reactivity to existing catalytic carboxylation reactions. This multiple C–C bond-forming reaction could be a useful tool for effecting otherwise inaccessible processes with CO2 as a coupling partner.
- Catalytic Intermolecular Dicarbofunctionalization of Styrenes with CO2 and Radical Precursors,
Veera Reddy Yatham, Yangyang Shen, Ruben Martin,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201706263