The greenhouse gas CO2 can be electrochemically reduced to give valuable products, using electricity sourced from renewable energies. Silver-based catalysts are highly active in CO2 electroreduction, but require a high overpotential, which makes the process economically impractical.
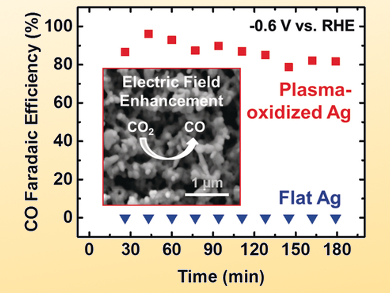
Beatriz Roldan Cuenya, Ruhr University Bochum and Fritz-Haber Institute of the Max Planck Society, Berlin, both Germany, Jan Rossmeisl, University of Copenhagen, Denmark, and colleagues have prepared silver foils with a low overpotential and a maximum CO Faradaic efficiency of 90 % at –0.6 V vs. a reversible hydrogen electrode (RHE).
The catalysts were pretreated with low-pressure O2 plasma to increase the roughness of the surface as well as the number of subsurface oxygen species. The team used scanning electron microscopy (SEM) and X-ray spectroscopy techniques to correlate improvements in catalytic performance with surface defects.
Density functional theory (DFT) calculations revealed that electric field effects on the defect-rich surface promote the reduction of CO2 to CO at low overpotentials. The study identifies surface defect sites as a key factor in lowering the thermodynamic barrier of the electrochemical reduction of CO2 to CO.
- Enhanced Carbon Dioxide Electroreduction to Carbon Monoxide over Defect-Rich Plasma-Activated Silver Catalysts,
Hemma Mistry, Yong-Wook Choi, Alexander Bagger, Fabian Scholten, Cecile S. Bonifacio, Ilya Sinev, Nuria J. Divins, Ioannis Zegkinoglou, Hyo Sang Jeon, Kim Kisslinger, Eric A. Stach, Judith C. Yang, Jan Rossmeisl, Beatriz Roldan Cuenya,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201704613