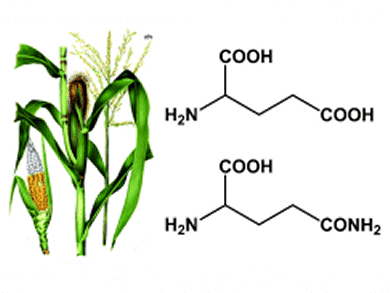
The chemical industry is in transition from a petrochemical based toward a more biobased industry. Succinonitrile, produced commercially from acrylonitrile and hydrogen cyanide, is the precursor of 1,4-diaminobutane (DAB, putrescine) in the industrial production of the polyamides Stanyl® and EcoPaXX®. The volume of DAB produced in Europe each year is estimated to be approximately 10,000 t.
Tijs M. Lammens, Wageningen University, The Netherlands, and colleagues describe the synthesis of biobased succinonitrile from glutamic acid and glutamine, amino acids that are abundantly present in many plant proteins.
Synthesis of the intermediate 3-cyanopropanoic amide was achieved from glutamic acid 5-methyl ester in an 86 mol% yield and from glutamine in a 56 mol% yield. 3-Cyanopropanoic acid can be converted into succinonitrile, with a selectivity close to 100% and a 62% conversion, by making use of a palladium(II)-catalyzed equilibrium reaction with acetonitrile.
Thus, a new route to produce biobased 1,4-diaminobutane has been discovered.
- Synthesis of Biobased Succinonitrile from Glutamic Acid and Glutamine,
Tijs M. Lammens, Jerome Le Notre, Maurice C. R. Franssen, Elinor L. Scott, Johan P. M. Sanders,
ChemSusChem 2011.
DOI: 10.1002/cssc.201100030