Many synthetically important metal-catalyzed organic transformations of alkynes involve metal vinylidene complexes. In the case of terminal alkynes, tautomerization generally generates mono-substituted vinylidene ligands, whose structures are well established. The straightforward preparation of disubstituted vinylidene complexes, in contrast, still remains a challenge. Such substructures are frequently found in bioactive compounds.
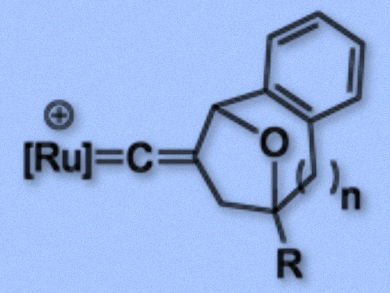
Ying-Chih Lin and colleagues, National Taiwan University, Taipei, have developed a cyclization reaction which uses aromatic propargyl acetates, tethered to epoxides, to obtain disubstituted vinylidene complexes in good to excellent yields. In the prepared complexes, the Cβ atom of the vinylidene is incorporated into a ring structure (example pictured). The team converted the aromatic propargyl acetates using the ruthenium complex Cp(PPh3)2Ru (Cp = η5-C5H5).
Intricate six-, seven- and eight-membered rings with oxo-bridges fused to an aromatic group were readily prepared in two steps. The first step involves a C–C bond formation between the propargyl unit and one of the carbon atoms of the epoxide ring, followed by a C–O bond formation between the epoxide oxygen and the propargyl unit. If the epoxide is transformed into a bromohydrin by dilithium tetrabromocuprate before the reaction, the cyclization of the bromohydrins with the propargyl unit can be performed in a single step.
- Cyclization Reactions of Aryl Propargyl Acetates with Tethered Epoxide Induced by Ruthenium Complex,
Yi-Jhen Feng, Yi-Hsin Chen, Shou-Ling Huang, Yi-Hung Liu, Ying Chih Lin,
Chem. Asian J. 2017.
DOI: 10.1002/asia.201701070