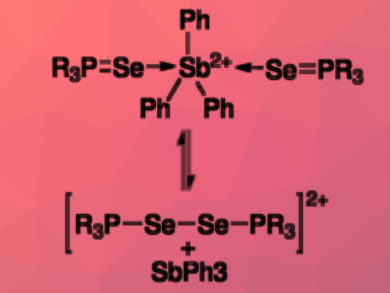
The chemistry of heavier p-block elements can have analogies to the coordination chemistry of transition metals. Ph3Sb(OTf)2 (OTf = triflate), for example, can form complexes with donor ligands featuring O or N atoms. In contrast, when phosphines are used as ligands, the antimony is reduced and diphosphonium dications are formed in a P–P coupling reaction.
Neil Burford, University of Victoria, Canada, and colleagues have discovered an analogous reaction of Ph3Sb(OTf)2 with trialkylphosphine selenides R3PSe (R = Me, iPr). The team used 31P{1H} NMR spectroscopy to monitor the process. They hypothesize that the reactions proceed via cationic coordination complexes of the type [Ph3Sb(R3PSe)2]2+ (pictured). From these complexes, diphosphonium diselenides are reductively eliminated.
The observed redox reaction is reversible: when Ph3Sb is added to the diphosphonium diselenides, the original complex is reformed in an oxidative addition.
- Reversible Oxidative Se−Se Coupling of Phosphine Selenides by Ph3Sb(OTf)2 ,
Maximilian J. Poller, Neil Burford, Konstantin Karaghiosoff,
Chem. Eur. J. 2017.
DOI: 10.1002/chem.201704753