Hydrogen peroxide (H2O2) is important for the regulation of a number of biological processes and is known for its cytotoxicity. The cellular damage inflicted by H2O2 has been linked to the initiation and progression of diseases such as Alzheimer’s, Parkinson’s, and cancer. However, H2O2 has also been found to act as a eukaryotic signal transducer, meaning that it could have positive or negative effects depending on the amount present and the cell or tissue type. Therefore, the ability to determine and quantify the amount of H2O2 present in a number of organs is of great importance.
Hwan Myung Kim, Ajou University, Suwon, Republic of Korea, and colleagues have synthesized a two-photon ratiometric fluorescent probe with a boronate-based carbamate leaving group as a reactive H2O2 trigger and 6-(benzo[d]thiazol-2’-yl)-2-(N,N-dimethylamino)naphthalene as the fluorophore. The reaction between the boronate group and H2O2 causes the probe to exhibit a fluorescent color change (from blue to yellow).
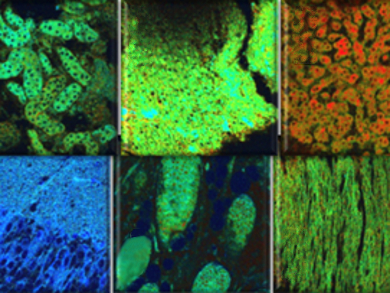
The probe gives a very clear color change and is highly sensitive and stable over a biologically relevant pH range. Combining this probe with two-photon ratiometric microscopy, the researchers were able to quantify endogenously produced cytosolic H2O2 levels in live cells as well as various rat organs. It was observed that the amount of H2O2 in organ tissues gradually increases in the order of brain, kidney, skin, heart, lung, and liver. This demonstrates that the new probe could be useful in clarifying the biological role of H2O2 and that it could also be used to diagnose certain diseases.
- A Two-Photon Ratiometric Fluorescent Probe for Imaging of Hydrogen Peroxide Levels in Rat Organ Tissues,
Chang Su Lim, Myoung Ki Cho, Mi Yeon Park, Hwan Myung Kim,
ChemistryOpen 2017.
DOI: 10.1002/open.201700155