The ionic radii of ionized main group elements (in pm). Cations have smaller radii and anions larger radii than their corresponding atoms.
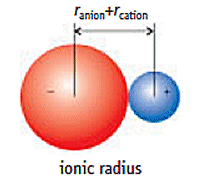
The ion radius increases within a group. In each additional period a new shell is occupied with electrons.
The ionic radius decreases within a period – but only within the cations. Anions are larger; here the radius also decreases within a period. This is due to the fact that the nuclear charge number (= proton number) increases and thus the attractive force of the atomic nucleus on the valence electrons becomes greater.
.gif)
-
See also: Atomic Radii of the Elements >>>
Taken from the book:
 World of the Elements, Elements of the World
World of the Elements, Elements of the World
Hans-Jürgen Quadbeck-Seeger
Wiley-VCH Verlag GmbH & Co, KGaA, Weinheim, 2007
Print ISBN: 9783527320653, Online ISBN: 9783527611577
DOI: 10.1002/9783527611577