Transmuting elements is not possible with chemical transformations. However, using the concept of “electronic transmutation”, aluminum can be made to act similar to silicon. Silicon hydrides are known to have homodinuclear double bonds, such as that in Si2H4. The Al=Al double bond has been otherwise notoriously difficult to synthesize.
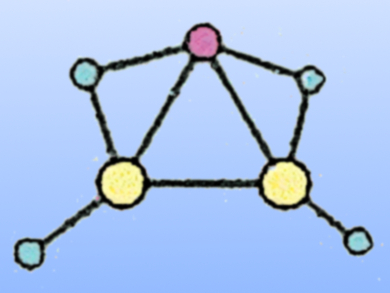
Kit H. Bowen, Johns Hopkins University, Baltimore, MD, USA, and colleagues have generated LiAl2H4– by coating an aluminum rod with a thin layer of LiAlH4 powder, and then ablating it with a pulsed laser beam. The resulting anions were characterized using mass spectrometry and photoelectron spectroscopy. Due to the donation of electrons from lithium to aluminum, the Al2H42– kernel in the LiAl2H4– cluster is both isoelectronic and isostructural to Si2H4, and an Al=Al double bond is observed.
The team used quantum chemical calculations to examine more than 10,000 possible isomers of this cluster and discovered that the global minimum (pictured, Al in yellow, H in blue, Li in red) has an Al=Al double bond and is in excellent agreement with the measured spectrum. Using similar electronic transmutation strategies, other exotic chemical bonding situations and compounds could be realized.
- On the Existence of Designer Al=Al Double Bond in the LiAl2H4– Cluster via Electronic Transmutation,
Katie A. Lundell, Xinxing Zhang, Alexander I. Boldyrev, Kit H. Bowen,
Angew. Chem. Int. Ed. 2017.
DOI: 10.1002/anie.201710338