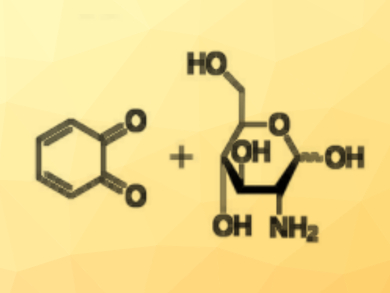
Hydrogels containing redox-active centers can be useful because they can transport both small molecules and electrons. Chitosan-based redox hydrogels, for example, are useful in medicine due to their biocompatibility. They can be produced by an electrochemical oxidation of catechol in the presence of chitosan. The oxidized catechol reacts with amine groups in chitosan (pictured) and acts as a crosslinker.
Nathan S. Lawrence, University of Hull, UK, Adrian Fisher, University of Cambridge, UK, and colleagues have studied the electrochemical oxidation of catechol in the presence of the chitosan base unit glucosamine or chitosan itself to understand and optimize the process. The team performed voltammetric measurements using a glassy carbon working electrode, a platinum counter electrode, and a saturated calomel reference electrode.
The researchers were able to observe the progress of the reaction and the degree of crosslinking using the voltammetric data. They found that the reaction follows a so-called EC mechanism, which means it involves an electron transfer (the oxidation of catechol), followed by a chemical reaction (the polymerization). The product is produced as a thin film on the electrode surface.
- Electrochemically Initiated Cross Linking of Chitosan,
Feng Zheng, Nathan S. Lawrence, Robert S. Hartshorne, Adrian Fisher,
ChemElectroChem 2017.
https://doi.org/10.1002/celc.201701303