Real-time analysis is a goal that has to be achieved to prevent pollution, as reported in the 11th of the 12 principles of green chemistry by Anastas and Warner. In agreement with the principles, the use of an organic solvent has to be avoided.
There has been growing interest in the use of hydrogen peroxide as an effective oxidant. It is considered environmentally sustainable because its active oxygen content is the highest after dioxygen, its by-product is water and its low cost makes it interesting for industrial applications. H2O2 is kinetically inert and, therefore, requires the use of a catalyst to be synthetically interesting. Different procedures involving catalysts/ILs and H2O2 have already been studied.
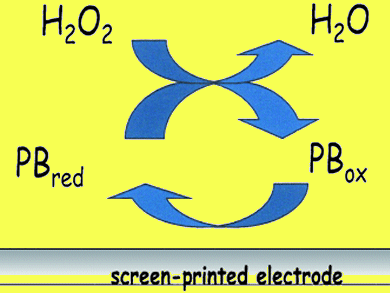
Fabian Arduini and colleagues, University of Rom, Italy, developed an electrochemical method to monitor the amount of H2O2 as a function of time in an organic oxidation reaction. The system is the first fast and efficient electroanalytical method to be applied to a continuous, in situ detection of H2O2 in organic oxidation reactions. It allows monitoring the real-time consumption of H2O2 with a cost-effective, portable and easy to use instrument and without the use of molecular solvents, according to green chemistry guidelines.
The organic reaction was carried out by using H2O2 as a primary sustainable oxidant in the presence of a catalyst, [VO(salophen)]TfO. This allowed to substitute stoichiometric processes and to use ILs as a green alternative to common volatile molecular solvents.
- Real-Time Monitoring of Hydrogen Peroxide Consumption in an Oxidation Reaction in Molecular Solvent and IonicLiquids by a Hydrogen Peroxide Electrochemical Sensor ,
Daniela Sordi, Fabiana Arduini, Valeria Conte, Danila Moscone, Giuseppe Palleschi,
ChemSusChem 2011.
DOI: 10.1002/cssc.201000386