The use of fluoroalkylseleno groups in catalysis has received increasing interest in recent years because of the potential to modify characteristics of molecules, such as lipophilicity, conformational changes, and electronic properties.
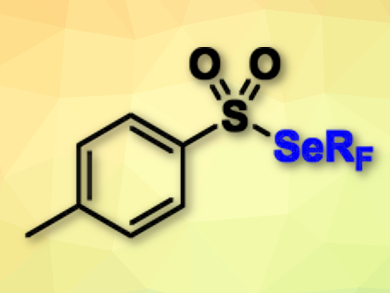
Thierry Billard, Anis Tlili, University of Lyon, France, and colleagues have achieved a direct fluoroalkylselenolation of terminal alkynes for the first time. The team used the shelf-stable electrophilic reagent trifluoromethyl tolueneselenosulfonate (pictured). A combination of cupric acetate and tetramethylethylenediamine turned out to be the best catalytic system to perform the trifluoromethylselenolation reactions at room temperature in the presence of Cs2CO3 as a base with toluene as the solvent.
The reaction shows high functional-group tolerance. Aliphatic compounds with a directing oxygen atom allow the regio- and stereoselective addition of TsSeCF3 on the triple bond, yielding new vinyl sulfone compounds. Interestingly, higher fluorinated homologues were also tolerated. The use of shelf-stable electrophilic reagents for trifluoromethylselenolation removes the need for stoichiometric copper salt sources and could offer new pathways in such reactions.
- Exploring the Reactivity of Trifluoromethyl Tolueneselenosulfonate with Alkynes under Copper Catalysis,
Clément Ghiazza, Vincent Debrauwer, Thierry Billard, Anis Tlili,
Chem. Eur. J. 2018, 24, 97–100.
https://doi.org/10.1002/chem.201705231