Iron-peroxo species are formed along the catalytic cycle of nonheme iron proteins involved in oxidative processes. An intense debate exists on whether such species are directly responsible for the reactivity or are merely a precursor of a metal-oxo reactive species.
Manuel G. Basallote, Universidad de Cádiz, Spain, Josep M. Luis, Miquel Costas, and Anna Company, Universitat de Girona, Spain, Lawrence Que Jr., University of Minnesota, Minneapolis, USA, and colleagues have characterized a novel iron(III)-hydroperoxo species with a nitrogen-based tetradentate ligand, [FeIII(OOH)(MeCN)(PyNMe3)]2+. This compound was generated by the reaction of its ferrous precursor [FeII(CF3SO3)2(PyNMe3)] with hydrogen peroxide at low temperatures.
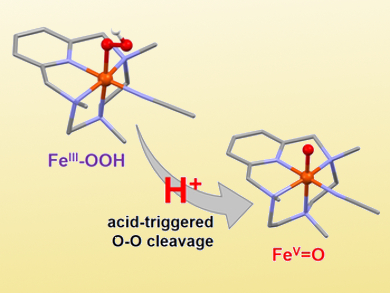
The iron(III)-hydroperoxo species itself cannot be used for the oxidation of C–H bonds. However, the addition of triflic acid causes an enhancement in its reactivity and allows the stereospecific oxidation of unactivated C–H bonds. Stopped-flow analysis and reactivity studies, together with density functional theory (DFT) calculations, suggest that a high valent metal-oxo species is formed upon protonation and heterolytic cleavage of the O–O bond (pictured).
Interestingly, in the heme enzyme cytochrome P450, the protonation of a peroxo moiety precedes the generation of the reactive high-valent iron-oxo species. Thus, this work suggests that a similar stepwise process can take place in nonheme systems.
- Acid-Triggered O−O Bond Heterolysis of a Nonheme FeIII(OOH) Species for the Stereospecific Hydroxylation of Strong C−H Bonds,
Joan Serrano-Plana, Ferran Acuña-Parés, Valeria Dantignana, Williamson N. Oloo, Esther Castillo, Apparao Draksharapu, Christopher J. Whiteoak, Vlad Martin-Diaconescu, Manuel G. Basallote, Josep M. Luis, Lawrence Que, Miquel Costas, Anna Company,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201704851