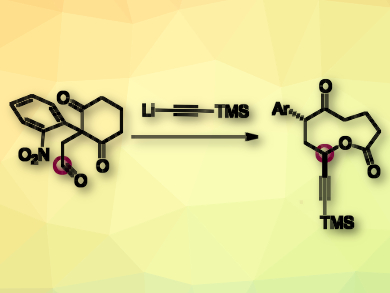
How can a lactone be prepared without a lactonization step? There are many creative answers to this question. The ring expansion of 1,3-cyclohexanedione is one example, but so far, 9-membered lactones have been difficult to obtain because of strain within the framework.
Michaël De Paolis, Normandie Université, Rouen, France, and colleagues have discovered a route to 9-membered lactones. The team used an anionic fragmentation of 2-nitrophenyl-1,3-cyclohexanediones which contain an electrophilic appendage such as an aldehyde or an epoxide (pictured). The reaction is initiated by a nucleophile. The mechanism proceeds in a domino-like fashion. The negative charge brought by the nucleophile is transferred from one heteroatom to a remote heteroatom, thus provoking the ring expansion.
This strategy provides access to lactones with a fused indole unit and a range of functional groups (alkyne, alkene, ester, nitrile, bromide, and azide). As they are relatively rigid and large, these lactones may interact with biological hosts. Since natural products combining medium-sized lactone and indole motifs are rather uncommon, this combination of the two is expected to be of interest in the molecular biosciences.
- Domino Ring Expansion: Regioselective Access to 9-Membered Lactones with a Fused Indole Unit from 2-Nitrophenyl-1,3-cyclohexanediones,
David Reyes Loya, Alexandre Jean, Morgan Cormier, Catherine Fressigné, Stefano Nejrotti, Jérôme Blanchet, Jacques Maddaluno, Michaël De Paolis,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201705645