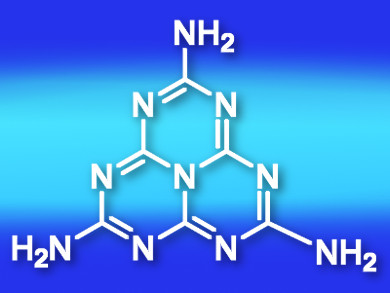
Graphitic carbon nitride (g-CN) is a potential metal-free photocatalyst. Its photocatalytic mechanism is proposed to involve its heptazine ring. Melem (1,3,4,6,7,9,9b-heptaazaphenalen-2,5,8-triamin; pictured) is the simplest heptazine-based compound. It is one of the condensation products of melamine. g-CN is the polymeric product of melem. Studies on the photophysics of melem can help researchers understand the photocatalytic mechanism of heptazine-based materials.
Rong Lu, Anchi Yu, Renmin University of China, Beijing, and colleagues have prepared melem and then gradually purified it. The researchers characterized the unpurified and purified melem using X-ray diffraction (XRD), infrared (IR) spectroscopy, elemental analysis, UV/Vis diffuse reflectance and fluorescence spectroscopy, as well as fluorescence decay lifetime measurements.
The team found that the condensation of melamine to melem causes stronger photoluminescence, whereas the condensation of melem to g‐CN causes weaker photoluminescence. In addition, a mixture of the monomer melem and its higher condensates is more easily obtained during the preparation of melem. Higher condensates of melem in the product mixture significantly affect the photophysical properties. The photocatalytic hydrogen evolution of melem has also been measured. It was found that the monomer melem has negligible photoinduced water-splitting activity.
- Photophysics and Photocatalysis of Melem: A Spectroscopic Reinvestigation,
Anchi Yu, Jing Wen, Ruiyu Li, Rong Lu,
Chem. Asian J. 2018.
https://doi.org/10.1002/asia.201800186