Chemical reactions involving carbon-centered radicals are highly attractive due to the mildness of their conditions, their high chemoselectivity, the ease of radical generation, and the ease of C–C bond formation. Traditionally, radical reactions are carried out as chain processes that proceed under substrate control. The alternative process of carrying out radical reactions through transition-metal catalysis under reagent control is not as well-established.
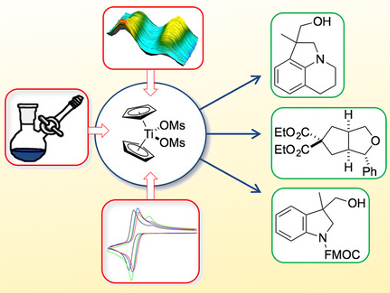
Andreas Gansäuer, University of Bonn, Germany, Robert A. Flowers II, Lehigh University, Bethlehem, PA, USA, and colleagues have studied the efficiency of titanocene catalysts (Cp2TiX) in the synthesis of a number of pharmaceutically relevant heterocycles, such as indolines, tetrahydroquinolines, and tetrahydrofurans, using radicals as key intermediates. The team synthesized titanocenes with sulfonate and carboxylate ligands rather than the usual halides. Kinetic investigations and cyclic voltammetry were used to characterize the complexes’ catalytic properties.
The screening showed that titanocene(III) mesylate (Cp2TiOMs; pictured) was both the most stable and the most selective catalyst. This is because it constitutes the best compromise between the required redox properties and low Lewis acidity.
These results demonstrate that the redox properties and the Lewis acidity of titanocene complexes and, hence, their ability to perform oxidative additions and reductive eliminations in single-electron steps, can be very easily tailored by their inorganic counter ions. This approach should have a strong impact on the future development of transition-metal-catalyzed radical reactions.
- Cp2TiX Complexes for Sustainable Catalysis in Single-Electron Steps,
Ruben B. Richrath, Theresa Olyschläger, Sven Hildebrandt, Daniel G. Enny, Godfred D. Fianu, Robert A. Flowers, Andreas Gansäuer,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201705707