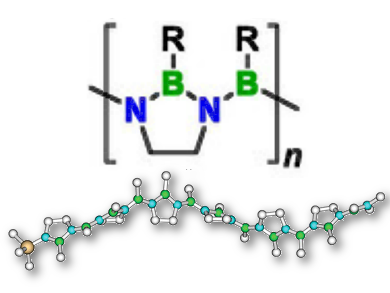
Inorganic polymers, such as silicones, often show useful properties and functions that complement those of organic macromolecular materials. Poly(iminoborane)s (PIBs) are main group polymers with a B=N backbone, formally isoelectronic to polyacetylene. Surprisingly, such species have not been synthesized and convincingly characterized so far.
Holger Helten and colleagues, RWTH Aachen University, Germany, have filled this gap in inorganic macromolecular chemistry. The major obstacle to the synthesis of PIBs was the competing facile formation of borazines (i.e., cyclic iminoborane trimers). This was overcome by linking the adjacent nitrogen atoms of the monomer by an ethylene bridge. The first well-defined oligo- and poly(iminoborane)s were obtained by a novel Si/B exchange polycondensation approach. An alternative synthetic route uses a dormant monomer that is activated for polymerization by an acid.
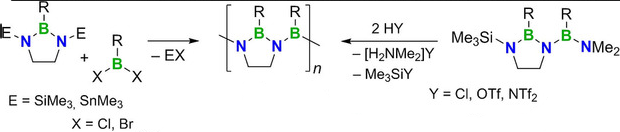
The properties of the new materials are effectively tuned by variation of their side groups, thus showing great potential for applications in materials science. Preliminary pyrolysis experiments suggest their possible use as precursors to valuable inorganic solid-state materials.
- Cyclolinear Oligo- and Poly(iminoborane)s: The Missing Link in Inorganic Main-Group Macromolecular Chemistry,
Ozan Ayhan, Nicolas A. Riensch, Clemens Glasmacher, Holger Helten,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201705913