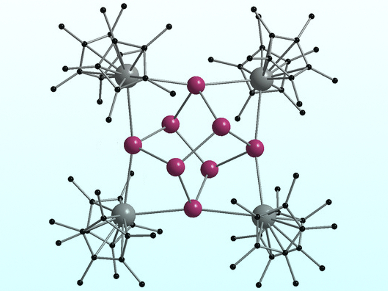
Zintl ions (anionic clusters of main-group elements) can react with rare-earth metals to produce molecular complexes of fundamental interest. Such complexes have been prepared from polypnictide (group 15) Zintl ions, with the exception of antimony, which is unreactive in its elemental form.
Peter W. Roesky, Karlsruhe Institute of Technology (KIT), Germany, and colleagues have prepared two reactive sources of antimony by reducing SbCl3: Sb0 nanoparticles and an Sb/Hg amalgam. The strong samarium reducing agent [Cp*2Sm] (Cp* = 1,2,3,4,5-pentamethylcyclopentadienyl) was then used to activate the Sb sources to produce molecular polystibides containing the f element Sm. The complexes were characterized using single-crystal X-ray crystallography.
The team isolated ternary Sm/Sb/Hg cluster reaction intermediates when the Sb/Hg amalgam was reduced at 60–70 °C, whereas a reaction temperature of 120 °C produced the polystibide complex [(Cp*2Sm)4(μ4,η2:2:2:2-Sb8)] (pictured). Sb0 nanoparticles generated the polystibide complex exclusively. According to the researchers, this complex is the largest molecular f-element polystibide ever isolated.
- Samarium Polystibides Derived from Highly Activated Nanoscale Antimony,
Christoph Schoo, Sebastian Bestgen, Alexander Egeberg, Svetlana Klementyeva, Claus Feldmann, Sergey N. Konchenko, Peter W. Roesky,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201802250