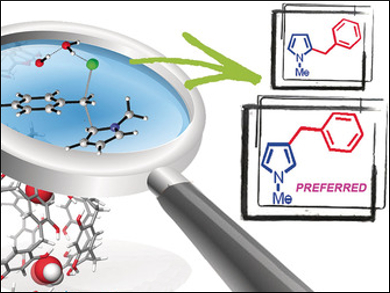
A hexameric capsule can be formed in solution by the self-assembly of six resorcinarene molecules with eight water molecules, driven by 60 hydrogen bonds. The nanoconfined environment inside the capsule is large enough to accommodate molecules. It can be used to accelerate the rate of some organic reactions.
Margherita De Rosa, Antonio Rescifina, Carmine Gaeta, and Placido Neri, Università di Salerno, Italy, and colleagues have shown that this hexameric capsule can accelerate a common electrophilic aromatic substitution, a Friedel–Crafts benzylation of several arenes and heteroarenes with benzyl chloride.
The catalytic effect is caused by the bridging water molecules of the capsule and benzyl chloride, which are able to polarize the C–X bond through hydrogen-bonding interactions. The reaction inside the capsule does not need strong Lewis acids, such as AlCl3. It is conducted simply by mixing the benzyl halide, the aromatic substrate, and resorcinarene in non-anhydrous chloroform (previously saturated with water).
N‐methylpyrrole is preferentially benzylated in the unusual β‐position. The fine supramolecular control means that the less π-nucleophilic mesitylene was benzylated faster than 1,3-dimethoxybenzene because of its stronger affinity for the capsule.
According to the researchers, using the hydrogen-bond donor ability of bridging water molecules could offer new pathways in supramolecular catalysis.
- Mild Friedel-Crafts Reactions inside a Hexameric Resorcinarene Capsule: C–Cl Bond Activation through Hydrogen Bonding to Bridging Water Molecules,
Pellegrino La Manna, Carmen Talotta, Giuseppe Floresta, Margherita De Rosa, Annunziata Soriente, Antonio Rescifina, Carmine Gaeta, Placido Neri,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201801642