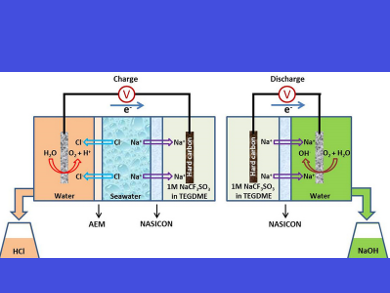
Youngsik Kim and colleagues, Ulsan National Institute of Science and Technology (UNIST), Republic of Korea, have developed the first rechargeable seawater desalination battery system, which integrates an electrochemical battery, seawater desalination, and acid‐alkali production in one system. The system consists of two parts:
- The charging part is composed of an anode (hard carbon electrode in organic electrolyte), natural seawater, and a cathode (Pt/C electrocatalyst in DI water). A NASICON ceramic electrolyte (Na3Zr2Si2PO12) separates the anode from the seawater, and an anion exchange membrane (AEM) separates the seawater from the cathode.
- The discharging part includes an anode (hard carbon electrode in organic electrolyte) and a cathode (Pt/C electrocatalyst in DI water) separated by a NASICON ceramic electrolyte.
During the charging process, Na+ from the seawater is extracted and transported to the anode through the NASICON separator, and is then stored in the hard carbon. Cl− from the seawater is extracted and transported to the cathode side through the AEM, followed by combination with H+, produced in the electrochemical oxygen evolution reaction (OER) process: 2H2O→O2+4H++4e−, to form HCl acid.
When discharging, Na+ stored in the hard carbon is transferred to the cathode side through the NASICON separator, followed by combination with OH−, produced in the electrochemical oxygen reduction reaction (ORR) process: O2+2H2O+4e−→4OH−, to form NaOH alkali.
The charging and discharging profiles indicate the good rechargeability and cycling stability of the battery system. According to the researchers, thi is a promising technology for using natural seawater resources. Future research will focus on improving the system configurations, the electrocatalysts for OER/ORR, the Na+ ions storage materials, as well as the NASICON ceramic electrolyte and anion exchange membrane.
- A New Rechargeable Seawater Desalination Battery System,
Yanjun Zhang, Sirugaloor Thangavel Senthilkumar, Jeongsun Park, Jehee Park, Youngsik Kim,
Batteries Supercaps 2018.
https://doi.org/10.1002/batt.201800012