Asymmetrically catalyzed reactions provide access to chiral compounds that are essential in drug discovery. However, stereodivergent approaches to all possible stereoisomers of target compounds are rare, and are especially challenging where a stereogenic carbon center remote from an axis of chirality is required.
Scott J. Miller and colleagues, Yale University, New Haven, CT, USA, have discovered a stereodivergent strategy to all possible stereoisomers of a heterocyclic scaffold containing both point and axial chiral elements. The researchers use peptidyl copper complexes to remotely desymmetrize diarylmethines through an enantioselective C–N bond-forming cross-coupling reaction. A C–N chiral axis is created on treatment with chiral C2-symmetric (CPA) or phosphothreonine-derived (pThr) phosphoric acids, which catalyze atroposelective cyclodehydration to form benzimidazole diastereoselectively.
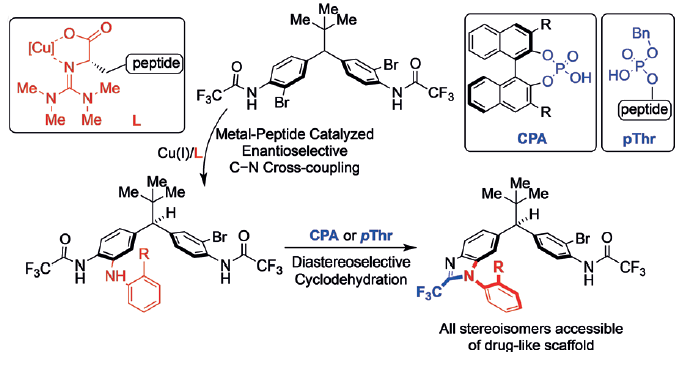
Tandem desymmetrization and cyclodehydration mediated by a combination of metal complexes and organocatalysts accesses all four stereoisomers of pharmaceutically relevant scaffolds. The team believes the method is applicable to synthesis of other stereochemically complicated systems.
- Divergent Control of Point and Axial Stereogenicity: Catalytic Enantioselective C−N Bond-Forming Cross-Coupling and Catalyst-Controlled Atroposelective Cyclodehydration,
Yongseok Kwon, Alex J. Chinn, Byoungmoo Kim, Scott J. Miller,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201802963