Porous membranes with high proton conductivity are important for energy-conversion schemes such as proton-exchange membrane fuel cells (PEMFCs) and water electrolyzers. The harsh operating conditions of these processes require materials that combine high chemical resistance with the ability to transport protons across a wide temperature range, ideally even below the freezing point of water.
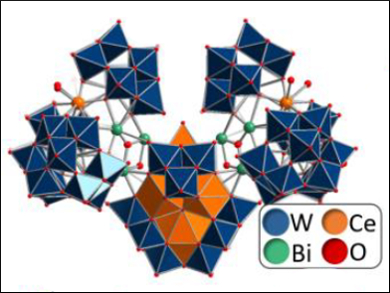
Jun-Wei Zhao, Henan University, China, Carsten Streb, University of Ulm, Germany, Yu-Fei Song, Beijing University of Chemical Technology, China, and colleagues have developed an aqueous synthesis in which commercial metal oxide precursors self-assemble in water to give giant bismuth cerium tungsten oxide molecules. The resulting compound, {[W14CeIV6O61]([W3Bi6CeIII3(H2O)3O14][B-α-BiW9O33]3)2}34– (pictured), was identified by single crystal X-ray diffraction.
The metal oxide clusters contain 104 metal centers and have dimensions of around 3.0 x 2.0 x 1.7 nm. The cluster anion is assembled around a central {Ce6} octahedron which is stabilized by several molecular metal oxide shells. Six trilacunary Keggin anions ([B‐α‐BiW9O33]9–) cap the superstructure and limit its growth.
In the crystalline state, the clusters aggregate into a chemically stable 3D porous molecular framework, which contains water-filled channels that enable high proton conductivity from –40 °C to +60 °C. According to the researchers, the findings highlight how molecular self-assembly can be combined with crystal design to access new high-performance porous molecular materials.
- Aggregation of Giant Cerium-Bismuth Tungstate Clusters into a 3D Porous Framework with High Proton Conductivity,
Li-Juan Chen, Qing Han, Jian-Cai Liu, Junwei Zhao, Carsten Streb, Yu-Fei Song,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201803649