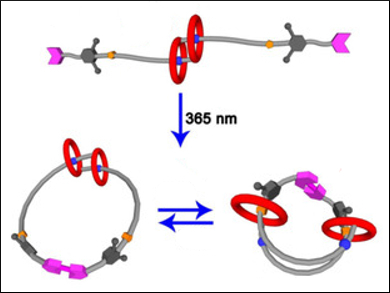
So-called [c2]daisy-chain rotaxanes (pictured top) are excellent candidates for building functional artificial molecular “muscles”. This is due to their unique ability of generating muscle-like contraction and extension motions upon external stimuli such as light or pH changes.
Most [c2]daisy-chain rotaxanes can perform a one-dimensional linear contraction/extension. One interesting strategy to achieve other modes of movement would be to transform [c2]daisy-chain rotaxanes into a “double-lasso macrocycle” (pictured bottom) by cyclization. However, there are very few examples of such systems.
Da-Hui Qu and colleagues, East China University of Science and Technology, have synthesized a [c2]daisy-chain-rotaxane-based double-lasso macrocycle. First, they prepared a precursor rotaxane which contained a [c2]daisy chain and two terminal coumarin groups using a copper(I)-catalyzed azide alkyne cycloaddition (CuAAC) reaction. Then, a [2+2]cycloaddition between the two coumarin groups was performed under UV light (λ = 365 nm), and a double-lasso macrocycle was obtained.
In the presence of acid/base stimuli, the [c2]daisy-chain unit showed reversible muscle-like extension/contraction (pictured bottom). The size of the double-lasso macrocycle could, thus, be tuned. This double-lasso macrocycle could be a useful addition to the family of mechanically interlocked molecules (MIMs).
- Light-Induced Cyclization of a [c2]Daisy-Chain Rotaxane to Form a Shrinkable Double-Lasso Macrocycle,
Si-Jia Rao, Xu-Hao Ye, Qi Zhang, Chuan Gao, Wen-Zhi Wang, Da-Hui Qu,
Asian J. Org. Chem. 2018.
https://doi.org/10.1002/ajoc.201800114



![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)