Very little is known about the chemistry of beryllium because of its extreme toxicity. The coordination mode around beryllium in proteins and the binding affinity towards the peptide are unknown because there have been no coordination compounds of beryllium with ligands bearing bio‐relevant functional groups. However, this would be important to understanding the biochemical mechanisms responsible for this toxicity.
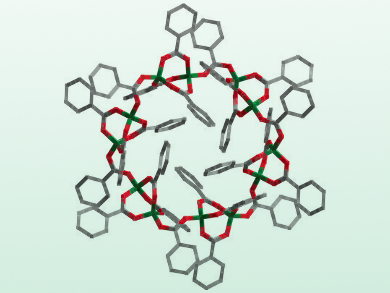
Magnus R. Buchner and Matthias Müller, Philipps-Universität Marburg, Germany, have performed a comprehensive study on these compounds. The researchers synthesized the first example of a nonbasic beryllium carboxylate (Be(PhCOO)2)12 (pictured), as well as the first beryllium adducts with aldehydes and non-deprotonated alcohols. Through single-crystal X-ray diffractometry and infrared (IR) spectroscopy in the solid state, as well as NMR spectroscopy in solution, the relative binding affinities of the beryllium cation to carboxylates, alcohols, aldehydes, and esters have been determined.
The team found that the binding affinities of Be2+ ions towards the functional groups are: carboxylate > alcohol > aldehyde > ester. Beryllium binding in proteins most likely occurs only via amino acid side chains and, for steric reasons, not directly to the peptide backbone. Also, non‐tetrahedral coordination modes at the beryllium atom seem very unlikely as even very large molecules are formed to facilitate fourfold coordinated beryllium.
This research provides the missing link between the relatively well-investigated aqueous coordination chemistry of beryllium and the biochemical and medicinal investigations into the mechanism of diseases associated with beryllium. Furthermore, these results could be important for the understanding of metal-triggered immune responses in general.
- Beryllium Complexes with Bio-Relevant Functional Groups: Coordination Geometries and Binding Affinities,
Matthias Müller, Magnus R. Buchner,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201803667