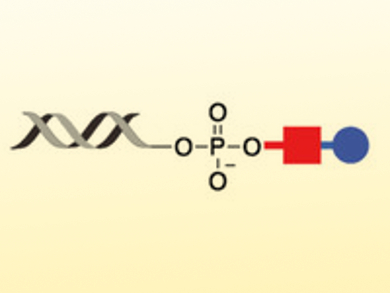
Methods to chemically incorporate photocleavable labels into nucleic acids in a defined and reversible manner could be very useful. Piyush K. Jain and Simon H. Friedman, University of Missouri, Kansas City, USA, have created a method that uses a novel reagent to allow the reversible modification of any nucleic acid, whether synthetic or naturally sourced, with a “clickable” and photocleavable group.
The team calls the reagent “Universal Light‐cleaved TerminI‐Modifying And TaggablE” (ULTIMATE). It works with all forms of RNA or DNA, as long as there is a terminal phosphate present. The reagent, a diazo‐di-methoxy nitro phenyl ethyl‐azide (pictured red), incorporates an azide group that can be modified by any “clickable” group (for example, a fluorophore, pictured blue). If needed, the modification can be removed using photolysis, revealing the original, unmodified nucleic acid.
According to the researchers, the ULTIMATE reagent should enable a wide range of studies of nucleic acids that would benefit from visualization and isolation.
- The ULTIMATE Reagent: A Universal Photocleavable and Clickable Reagent for the Regiospecific and Reversible End Labeling of Any Nucleic Acid,
Piyush K. Jain, Simon H. Friedman,
ChemBioChem 2018.
https://doi.org/10.1002/cbic.201800028