Magnesium plays a critical role in biochemistry. It stabilizes DNA structure and plays a key role in many enzymatic reactions. Many diseases of the heart, kidneys, and nervous system have been traced to misregulation of magnesium-ion concentrations. However, it is very difficult to measure magnesium concentrations in the serum in the presence of calcium. There are no chelating ligands that bind magnesium with sufficient selectivity over calcium to allow direct measurements when both ions are present in the millimolar range.
The o‐aminophenol‐N,N,N‐triacetic acid (APTRA) unit is the most widely used binding chelate for magnesium in the literature. It has been incorporated into many luminescent systems. Luminescent lanthanide(III) complexes offer a number of fundamental advantages over other systems. They have large pseudo‐Stokes’ shifts, sharp emission bands that are characteristic of each lanthanide ion, and a fine structure in their emission spectra that is sensitive to the nature of the metal coordination environment.
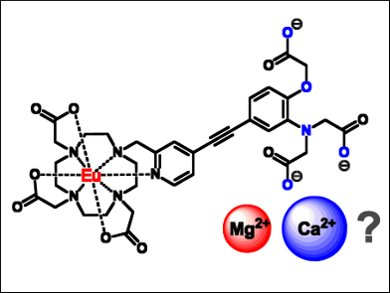
David Parker, Gareth Williams, and Edward Walter, Durham University, UK, have developed a series of luminescent europium(III) complexes (example pictured) in which an APTRA moiety is integrated into the sensitizing chromophore (APTRA = o‐aminophenol‐N,N,N‐triacetate; pyridylalkynylaryl and related bi‐aryl = chromophores). These ligands bind calcium and zinc ions weakly, without compromising magnesium affinity too much. Bound magnesium ions change the emission properties of the ligands, which can then be measured.
According to the researchers, their new lanthanide‐based APTRA complexes show enhanced selectivity towards magnesium-ion concentrations in serum compared with APTRA derivatives previously discussed in the literature. The method could be a basis for further work to enhance selectivity even more.
- APTRA-Based Luminescent Lanthanide Complexes Displaying Enhanced Selectivity for Mg2+,
Edward R. H. Walter, J. A. Gareth Williams, David Parker,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201800745