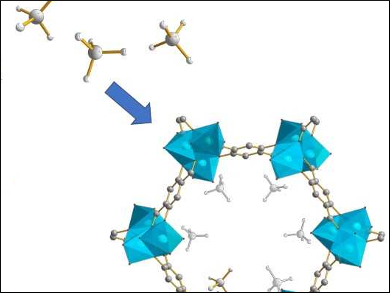
Methane, the main component of natural gas, has a low volumetric energy density that makes the storage of large amounts of energy challenging. Metal-organic frameworks (MOFs) are three-dimensional porous materials that have shown promise for methane capture and storage. To improve methane adsorption in MOFs, more detailed knowledge of methane locations, mobility, and MOF-methane interactions would be useful.
Yining Huang, The University of Western Ontario, Canada, and colleagues have used 2H solid-state nuclear magnetic resonance (SSNMR), single-crystal X-ray diffraction (SCXRD), and density functional theory (DFT) calculations to examine methane guests in four different MOFs: α-Mg3(HCO2)6, α-Zn3(HCO2)6, SIFSIX-3-Zn, and M-MOF-74 (M = Mg, Zn, Ni, Co; Mg-MOF-74 pictured above).
The results indicate that methane is highly mobile with a single adsorption site. SSNMR indicates freely tumbling methane rapidly exchanges with adsorbed methane, with NMR parameters closely related to binding strength. Calculations reveal that the methane adsorption is driven mainly by dispersive (i.e., van der Waals) forces, which are inversely related to MOF pore size.
According to the researchers, this characterization strategy could also be used to investigate methane within other MOFs and porous compounds and to assist the development of new materials for methane capture and storage.
- A Multifaceted Study of Methane Adsorption in Metal-Organic Frameworks by Using Three Complementary Techniques,
Yue Zhang, Bryan E. G. Lucier, Michael Fischer, Zhehong Gan, Paul D. Boyle, Bligh Desveaux, Yining Huang,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201800424