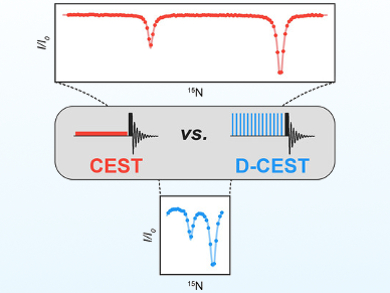
NMR spectroscopy methods are used for the study of excited protein states, which can play key roles in many biochemical processes. In particular, Chemical Exchange Saturation Transfer (CEST) is a powerful approach for studying minor, so-called “invisible” protein states in slow exchange with a major visible conformation. In a CEST experiment, each frequency in the region of interest is interrogated to one spectrum at a time. However, this simplicity is also a weakness of the method, as the recording of CEST profiles with enough points to cover the entire spectrum can be time-consuming.
Guillaume Bouvignies, École Normale Supérieure, PSL University, Sorbonne Université, CNRS, Paris, France, and colleagues have proposed a simple solution to this problem: a multi-site excitation scheme that allows multiple frequencies to be irradiated simultaneously. The team used the so-called “Delay Alternating with Nutation for Tailored Excitation” (DANTE) excitation scheme, which uses short, rectangular pulses (pictured right) to provide a frequency-selective excitation.
The researchers call their method D-CEST, short for DANTE-CEST. The developed approach significantly decreases acquisition times—by as much as an order of magnitude in some cases. It also extends the methodology to systems with small chemical-shift differences between exchanging states.
- Dramatic Decrease in CEST Measurement Times Using Multi-Site Excitation,
Tairan Yuwen, Lewis E. Kay, Guillaume Bouvignies,
ChemPhysChem 2018.
https://doi.org/10.1002/cphc.201800249