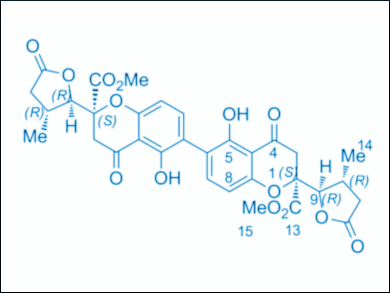
Natural products are important lead structures in the development of drugs and agrochemicals. Often, the desired compounds can only be obtained in small quantities from natural sources, which makes the development of a synthetic approach necessary. A rather new group of natural products with high bioactivity are dimeric chromanone lactones, which have been isolated from different fungi.
Lutz F. Tietze, University of Göttingen, Germany, and colleagues have performed the first enantioselective total synthesis of two members of this new group, the homo-dimeric blennolide H (pictured) and phomopsis H76 A. The team used a Wacker oxidation in the presence of a 3,3′-disubstituted 2,2′-bis(oxazolyl)-1,1′-binaphthyl (BOXAX) ligand to form the desired monomer with ee values of 99 %. A second key step is a Suzuki reaction of an intermediate monomeric iodide using Pd(OAc)2 as a catalyst, 2-dicyclohexylphosphino-2′,6′-dimethoxybiphenyl (SPhos) as a ligand, and bis(pinacolato)diboron (B2pin2), which gives the dimeric structure.
On the basis of the results, it was possible to revise the absolute configuration of one of the compounds and determine the relative and absolute stereochemistry of the other compound. Future work could focus on the synthesis of similar natural products, possibly with antifungal activity, and the determination of their bioactivity.
- Enantioselective Total Synthesis of Blennolide H and Phomopsis-H76 A and Determination of Their Structure,
Guillermo Valdomir, Soundararasu Senthilkumar, Dhandapany Ganapathy, Yun Zhang, Lutz F. Tietze,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201801323