High-valent late-transition-metal fluorides are among the strongest known oxidizing agents. IrF6 and PtF6, in particular, are used as high-potential oxidizers. However, there is a lack of data on the molecular structure and spectroscopic properties of low-valent iridium fluorides, and a systematic investigation that considers all possible iridium fluoride species had not been reported so far.
Sebastian Riedel, Freie Universität Berlin, Germany, and colleagues, have synthesized and characterized molecular iridium fluorides, combining matrix-isolation spectroscopy and theoretical studies. For the first time, the whole series of IrFn (n = 1-6) has been investigated. The team studied the photo-initiated defluorination of iridium hexafluoride (IrF6) in neon and argon matrices at 6 K. The photoproducts were characterized by infrared (IR) and UV-Vis spectroscopy as well as quantum-chemical calculations.
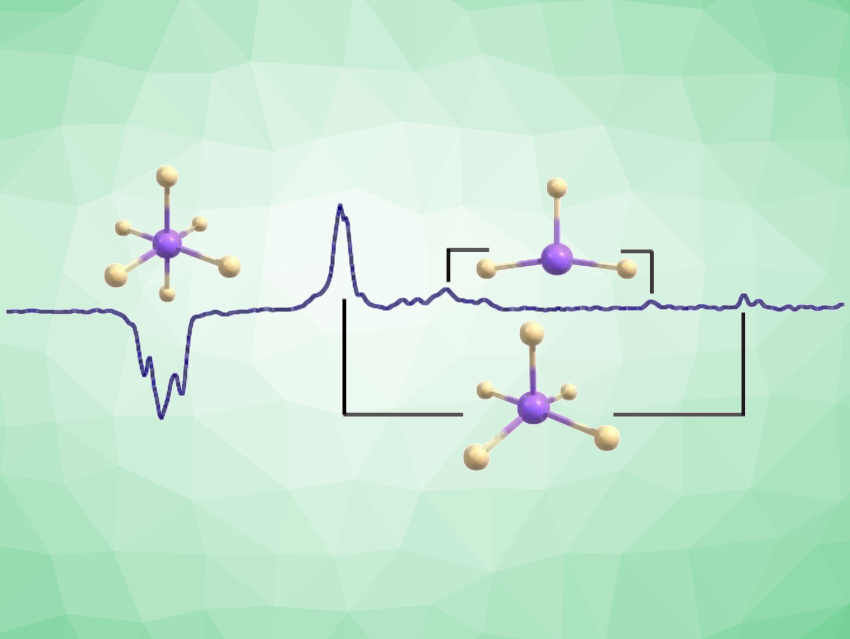
The primary photoproducts obtained from IrF6 after UV irradiation (λ = 365 nm) are iridium pentafluoride (IrF5) and iridium trifluoride (IrF3). Longer irradiation of the same matrix (λ = 278 nm) gave iridium tetrafluoride (IrF4) and iridium difluoride (IrF2) by Ir−F bond cleavage or F2 elimination. In addition, IrF5 can be transformed back to IrF6 by the addition of an F atom under blue-light irradiation (λ = 470 nm). Laser irradiation (λ = 266 nm) of IrF4 also generated IrF6, IrF5, IrF3, and IrF2. The team found that spin-orbit coupling effects in IrF5 lead to a triplet ground state with C4v symmetry—a rare example of a molecule in which spin-orbit coupling has a significant influence on the structure.
- Investigation of Molecular Iridium Fluorides IrFn (n = 1‐6): A Combined Matrix‐Isolation and Quantum‐Chemical Study,
Sebastian Hasenstab-Riedel, Yan Lu, Yetsedaw A. Tsegaw, Artur Wodyński, Lin Li, Helmut Beckers, Martin Kaupp,
Chem. Eur. J. 2022.
https://doi.org/10.1002/chem.202104005