Organic lithium-ion batteries (LIBs) are promising energy storage devices. However, small organic molecules are soluble in aprotic electrolytes and have an intrinsically low electric conductivity. An efficient strategy to improve these properties is to combine small organic molecules in polymeric units, which prevents dissolution. One disadvantage of this technique is that the use of polymer electrodes usually produces agglomeration, causing the inefficient use of the redox-active functional groups of the molecule.
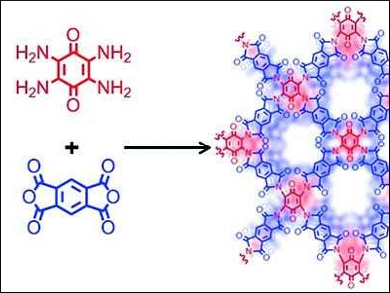
Fujun Li, Nankai University, Tianjin, China, and colleagues have synthesized a microporous covalent organic framework (COF) of poly(imide‐benzoquinone), polymerized on graphene, (PIBN-G) as a cathode material for lithium‐ion batteries. The COF is synthesized from tetramino‐benzoquinone and pyromellitic dianhydride (pictured). PIBN-G is a two-dimensional framework with micropores of around 1.5 nm. This allows for good diffusion and easy access of Li+ to the large number of redox-active carbonyl groups of the COF and promotes reversible electrochemical reactions.
According to the researchers, the strong interaction and charge transfer between PIBN molecules and graphene provide high electric conductivity. The PIBN-G electrodes have large reversible specific capacities of 271.0 mAh/g and 193.1 mAh/g at 0.1 C and 10 C, respectively. Batteries with PIBN-G cathodes retain more than 86 % of their capacity after 300 charge/discharge cycles. This work could be a basis for further investigations into the use of COFs in electrochemistry.
- A Microporous Covalent-Organic Framework with Abundant Accessible Carbonyl Groups for Lithium-Ion Batteries,
Zhiqiang Luo, Luojia Liu, Jiaxin Ning, Kaixiang Lei, Yong Lu, Fujun Li, Jun Chen,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201805540