About 30 % of human proteins are intrinsically disordered, i.e., they lack a well-defined 3D structure. Interactions with the solvent cause such proteins to maintain an extended and open form rather than a rigid, folded structure. Intrinsically disordered regions in proteins can possess many functions. They can, e.g., bind other proteins, interact with nucleic acids, or serve as scaffold proteins. They are also involved in signaling pathways and have important roles in protein regulation.
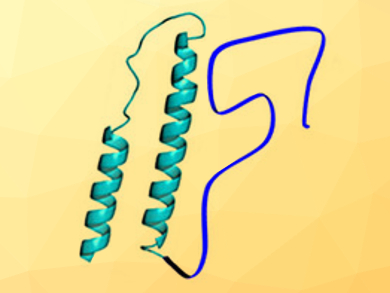
Assaf Friedler, Hebrew University of Jerusalem, Israel, and colleagues have used HIV-1 Rev (an essential protein for the regulation of the human immunodeficiency virus) as a model protein to study the interplay between structured and disordered domains in the same protein. HIV-1 Rev contains a structured N-terminal part and a disordered C-terminal part (pictured). The team used circular dichroism spectroscopy and size-exclusion chromatography to study different modified HIV-1 Rev proteins, each one with the disordered “tail” shortened by approximately ten additional amino acid residues.
The results showed that the disordered C-terminal domain has two sub-regions that carry out two distinct, complementary roles: regulating oligomerization of the protein and contributing to the protein’s stability. The researchers suggest that these functions are carried out by a delicate balance between charged and hydrophobic residues within the intrinsically disordered region. The team further proposes that intrinsically disordered regions in proteins should be divided to subdomains similarly to structured regions, rather than being viewed as long flexible stretches.
- Protein Regulation by Intrinsically Disordered Regions: A Role for Subdomains in the IDR of the HIV-1 Rev Protein,
Ofrah Faust, Dana Grunhaus, Odelia Shimshon, Eylon Yavin, Assaf Friedler,
ChemBioChem 2018.
https://doi.org/10.1002/cbic.201800192