Hydrogen is a clean-burning fuel that is currently produced from fossil sources. Generating hydrogen directly from water, driven by solar light, is a renewable alternative. Research in this area is focused on designing light-absorbing particles that use solar energy to drive the conversion catalytically. These systems are typically limited to anaerobic conditions due to the inhibiting effects of O2.
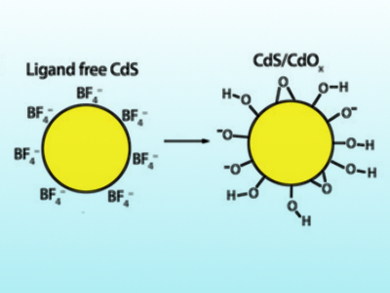
Erwin Reisner and colleagues, University of Cambridge, UK, have optimized cadmium sulfide (CdS) quantum dots (QDs) to generate hydrogen under aerobic conditions. CdS QDs are nanocrystals below 10 nm in diameter. The team synthesized capping-ligand-free CdS QDs by ligand-stripping of oleic acid-capped CdS QDs. The photocatalytic activity of the QDs was tested by combining the QDs with Co(BF4)2 as a co‐catalyst for the proton‐reduction reaction and EtOH as an electron donor under an air stream in a photoreactor that was irradiated with simulated sunlight. The production of H2 was analyzed at designated time intervals by gas chromatography (GC). The system reached the highest reported rate for photocatalysis driven by an organic oxidation reaction on CdS under comparable experimental conditions.
The results show that CdS quantum dots do not necessarily suffer from O2 inhibition, but can even be stabilized under aerobic conditions. According to the researchers, O2 prevents the over‐accumulation of metallic Cd and particle agglomeration—a key inactivation pathway of CdS—and, thus, provides the particles with higher stability. This strategy could help with the design of other, similarly active catalysts.
- Aerobic Conditions Enhance the Photocatalytic Stability of CdS/CdOx Quantum Dots,
David W. Wakerley, Khoa H. Ly, Nikolay Kornienko, Katherine L. Orchard, Moritz F. Kuehnel, Erwin Reisner,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201802353