Diazo compounds have a wide variety of applications in synthetic organic chemistry. Because diazo compounds have
a partial negative charge on the carbon connected to the diazo group, they can undergo formal C–C bond insertion reactions with carbonyl compounds (aldehydes or ketones) in the presence of Lewis acid catalysts. The reation is believed to proceed via nucleophilic addition of the diazo compounds to the carbonyl compounds followed by a rearrangement of the resulting zwitterionic intermediate. Most of these reactions involve monocarbonyls as electrophiles. 1,3-diketones are commonly used in organic synthesis, however, catalytic C–C bond insertion reactions of 1,3-diketones with diazo compounds remain unknown. The analogous reaction with 1,3-diketones gives C–H bond insertion products instead.
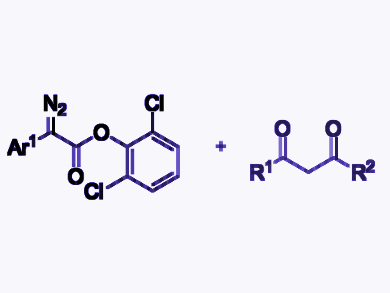
Shou-Fei Zhu, Nankai University, Tianjin, China, and colleagues have developed a protocol for the gold-catalyzed formal C–C bond insertion reaction of 2-aryl-2-diazoesters with 1,3-diketones (example substrates pictured). The team combined the diazoesters with the diketones in the presence of (ArO)3PAuNTf2 (Ar = 2,4-tBu2C6H3, Tf = trifluoromethanesulfonyl) as a catalyst and H3PO4 as a Brønstedt acid in CH2Cl2 at 25 °C. The desired products were obtained in good yields.
The reaction provides efficient access to polycarbonyl compounds with a quaternary carbon center. The aryl ester moiety plays a crucial role in the unusual chemoselectivity. The researchers propose a reaction mechanism involving the cyclopropanation of a gold carbenoid with an enolate and a ring-opening of the resulting donor–acceptor-type cyclopropane intermediate. This mechanism differs from that of the traditional Lewis-acid-catalyzed C–C bond insertion reaction of diazo compounds with monocarbonyl compounds, which features the rearrangement of a zwitterion intermediate as a key step as described above.
- Gold-Catalyzed Formal C-C Bond Insertion Reaction of 2-Aryl-2-diazoesters with 1,3-Diketones,
Yuan-Yuan Ren, Mo Chen, Ke Li, Shou-Fei Zhu,
Chem. Asian J. 2018.
https://doi.org/10.1002/asia.201800934