Superior Photocatalysts: Triazine-based Polymeric Frameworks
They are especially good photocatalysts for the production of hydrogen by splitting water with solar energy: covalent organic frameworks based on triazines. For this application, the frameworks need to be in a nice regular crystalline form. Scientists have introduced a simple method for synthesizing crystalline covalent triazine frameworks.
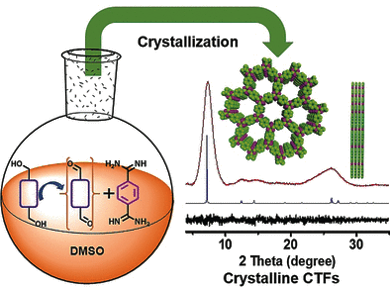
Triazines are comprised of heteroaromatic rings containing three carbon and three nitrogen atoms. Triazine-based polymeric frameworks are made by the polycondensation of two types of monomers, i.e., aromatic ring systems with two aldehyde groups and aromatic rings with two amine-containing groups. These terminal groups react with each other to form triazine rings and connect the monomers into two-dimensional web-like layers. Within the crystal, the polymer layers are precisely stacked so that the “webs” form channels passing all the way through the polymer scaffold.
Triazine frameworks are unfortunately hard to obtain as crystals because the carbon-nitrogen double bonds formed in the polycondensation are very strong. The stronger the bonds within the frameworks, the harder it is to crystallize such compounds. The usual crystallization process is an equilibrium reaction in which bonds are formed and then dissolved, allowing bigger crystals to form at the cost of smaller ones. Strong bonds do not break easily, so that many tiny crystals remain intact and are clumped into an amorphous powder.
New Strategy to Crystalline Covalent Triazine Frameworks
Researchers at Huazhong University of Science and Technology, Wuhan, China, Luoyang Normal University, Luoyang, China, and the University of Liverpool, UK, have introduced a new strategy to obtain highly crystalline triazine frameworks. Instead of an equilibrium reaction, they used an “open system”, where one of the two monomers is added to the system very slowly. This is possible because aldehydes can be formed during the reaction (in situ) by the oxidation of alcohols—a reaction whose speed can easily and reliably be controlled by temperature control. In this way, only very few crystallization nuclei are formed initially, which grow into larger and high-quality crystals as the reaction continues.
Starting with different alcohols, the team led by Bien Tan and Shangbin Jin was able to produce a series of covalent and crystalline triazine frameworks. The crystals demonstrated higher thermal stability than the smaller or non-crystalline triazine frameworks. Most importantly, however, their efficiency as photocatalysts for the production of hydrogen is very high. The higher photocatalytic activity is because of the significantly improved transport of charge carriers produced by irradiation within the regular crystal, and the absorption of a broader spectrum of light.
This novel and simple synthetic approach may be the starting point for the industrial production of crystalline triazine frameworks.
- Crystalline Covalent Triazine Frameworks by In Situ Oxidation of Alcohols to Aldehyde Monomers,
Manying Liu, Qi Huang, Shaolei Wang, Ziyong Li, Buyi Li, Shangbin Jin, Bien Tan,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201806664