Dynamic helical oligomers and polymers are mostly build of achiral repeating units, but with a chiral residue covalently or noncovalently bound to one chain end. This generates either a right-handed (P)-helix or a left-handed (M)-helix in a domino-like fashion. One of the most interesting features of dynamic helical oligomers and polymers is a long-range chiral information transfer.
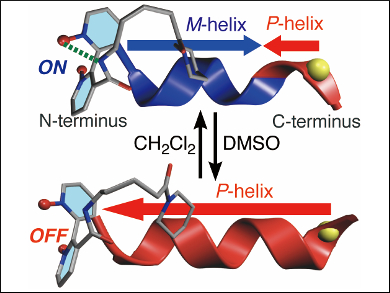
Akio Urushima, Naoki Ousaka, and Eiji Yashima, Nagoya University, Japan, have found a solvent-induced switching of the helix handedness of a lactam-bridged dynamic helical oligopeptide. It has different chiral units at both ends, e.g., an axially chiral unit at the N-terminus and an L-amino acid at the C-terminus (pictured). The handedness of the helix partially switches when the solvent is changed from CH2Cl2 to dimethyl sulfoxide (DMSO) or vice versa.
This effect is a result of competitive helix-inducing biases—like a tug-of-war between two chiral twisting forces generated from the two chiral terminal units. The chiral domino effect from the N-terminal unit can be switched on and off, as shown in the picture. The effect is triggered by the solvent‐induced breaking of a specific intramolecular hydrogen bond. These findings may provide a rational design strategy for chirality-switchable asymmetric catalysts, and could also contribute to understanding the mechanism of solvent-induced helix-inversion in other helical systems.
- Tug-of-War in a Dynamic Helical Peptide: Solvent-Induced Helix-Helix Transition of a Lactam-Bridged Peptide Composed of Point- and Axial Chiralities Remote from Each Other,
Akio Urushima, Naoki Ousaka, Eiji Yashima,
Chem. Asian J. 2018.
https://doi.org/10.1002/asia.201801111