Supramolecular polymers are important building blocks in living organisms and are of interest for the development of new materials. However, it is challenging to control self-assembly and gel formation on solid surfaces and interfaces.
Pol Besenius, University of Mainz, Germany, and Thomas M. Hermans, University of Strasbourg, France, and colleagues have developed a dendritic peptide conjugate that responds to pH changes due to its amphiphilic character. Below a pH value of 6, the benzene-1,3,5-tricarboxamide peptide amphiphile self-assembles into supramolecular fibers. At high pH, the monomer has several negative charges. The resulting electrostatic repulsion between the monomers prevents self‐assembly.
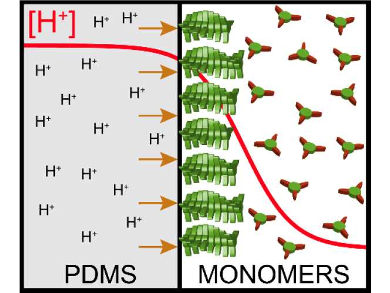
Using this pH dependence, the growth of a gel-like supramolecular layer was constrained to the surface of a poly(dimethyl siloxane) polymer (PDMS). The PDMS was pre-soaked in concentrated hydrochloric acid and acts as a proton releasing material (pictured).
Using stopped-flow experiments in a microfluidic device, the team analyzed the kinetics of the pH-regulated supramolecular polymerization and determined that release of protons from the polymeric bulk is slower than the nucleation rate of the supramolecular polymers. This leads to the progressive growth of the hydrogel outwards from the surface. The researchers believe the approach can be applied to other pH-responsive gelators and could be used to prepare a range of layered materials on solid substrates.
- Surface-Assisted Self-Assembly of a Hydrogel by Proton Diffusion,
Daniel Spitzer, Vincent Marichez, Georges J. M. Formon, Pol Besenius, Thomas M. Hermans,
Angew. Chem. Int. Ed. 2018, 57, 11349–11353.
https://doi.org/10.1002/anie.201806668