Natural enzymes have characteristic three-dimensional structures and specific catalytic activities. This structure–activity relationship is difficult to mimic with artificial organic polymers. The most widely explored biomimetic enzymes to date are organic polymers incorporating heavy or noble metals as their active sites. A Chinese team of scientists has now prepared single-chain polymeric nanoparticles with titanium as their active-site metal, which catalyzed asymmetric sulfoxidations in water.
Mimicking Enzymes
In natural enzymes, single chains of amino acids (or nucleotides in the case of ribozymes) fold into well-preserved three-dimensional structures containing active-site pockets where one and only one reaction can take place at a time. Mimicking enzymes fascinates scientists, both because it is extremely challenging to create such structure–activity relationships in polymers and because new catalytic processes could be developed for chemical industry.
To mimic enzymes, scientists have developed single-chain organic polymers that fold into nanoparticles of about the same size as their natural counterparts. However, the polymerization processes do not yet allow the incorporation of the building blocks in a sequential order. “This is still difficult to achieve. That’s why the polymers constituting the single-chain nanoparticles still have a statistical distribution of their building blocks,” says Christopher Barner-Kowollik who leads a team where single-chain polymeric nanoparticles are explored at the Queensland University of Technology, Brisbane, Australia, and the Karlsruhe Institute of Technology (KIT), Germany. Therefore, artificial single-chain polymers do not fold into the defined architectures displayed by natural enzymes. Depending on the solvent, they collapse into random coils.
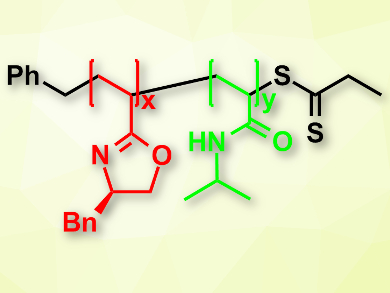
The challenge is to construct a defined and stable architecture for the active sites to promote reactions with high turnover rates. Rong Tan and his colleagues from Hunan Normal University, Changsha, China, have now reported a single-chain polymeric nanoparticle containing titanium(IV) ions in a coordination sphere with chiral oxazolines. It promoted the asymmetric sulfoxidation of various organic sulfides in water. Raising the temperature made the polymer insoluble and recoverable from the solution.
The Active Site And The Catalyst
Rong Tan and his co-workers chose titanium ions as the catalytic center because titanium ions coordinate to oxazolines and other useful ligands and could control and even lock the collapse of single-chain linear polymers in various solvents. Besides, complexes with titanium, as an abundant light metal, are currently heavily investigated catalysts in organic synthesis. The researchers proposed titanium coordination to chiral oxazolines would lead to three-dimensional structures and asymmetric induction in the catalytic reaction. The polymer backbone was constructed from acrylamide copolymers.
First, reversible addition–fragmentation chain transfer polymerization led to the random copolymer. Then a titanium reagent was added for coordination and folding. The single-chain nanoparticles catalyzed the oxidation of several aryl alkyl sulfides into chiral sulfoxides by hydrogen peroxide in water, and with high turnover rates. Heating above the lower critical solution temperature made the polymer insoluble, ready for decanting and reusing. The researchers argued that the hydrophobic oxazoline residues provided the coordination sphere to hold the titanium ion while, at the same time, balancing hydrogen peroxide access to avoid over-oxidation.
Applications
The scientists have synthesized a recoverable biomimetic polymer system for asymmetric catalysis, involving titanium as an active, light metal. “It is desirable to embed a variety of metal centers and test their possibilities for catalysis in these single-chain polymer nanoparticles,” Barner-Kowollik comments. “The aim of single-chain polymer nanoparticles research is, as a first step, to construct a homogeneous catalytic system that can be re-isolated by filtration,” Barner-Kowollik says. That has been achieved with heavy metals before, but here Tan and his team used titanium as a light metal for asymmetric synthesis and achieved for the first time high turnover rates.
A next step would be the tailoring of the catalytic pockets to achieve a higher turnover rate than with the catalyst alone. Future ideas relate to switchable catalysts by light or other triggers, properties that can be endowed by the polymer design.
- Titanium(IV)-folded single-chain polymeric nanoparticles as artificial metalloenzyme for asymmetric sulfoxidation in water,
Yaoyao Zhang, Weiying Wang, Wenqin Fu, Mingjie Zhang, Zhiyang Tang, Rong Tan, Donghong Yina,
Chem. Commun. 2018, 54, 9430–9433.
https://doi.org/10.1039/C8CC05590D