Reactions in which new C–C or C–X bonds are formed are of great importance in chemistry. Polymetallic species could be interesting catalysts for such reactions. In particular, when the catalysts are heterobimetallic pairs, the interchange of their ligands (transmetalation) seems to be a key process. Dative interactions between the metal centers may play a crucial role in the activity of these bimetallic systems, but they have seldom been characterized in depth.
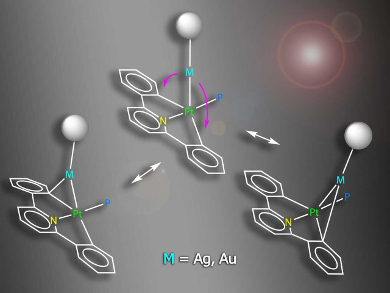
Antonio Martín, Universidad de Zaragoza, Spain, and colleagues have prepared, characterized, and studied four complexes containing Pt–Au or Pt–Ag bonds (example pictured), namely [(CNC)(PPh3)PtM(PPh3)]ClO4 (M = Au, Ag) and [{Pt(CNC)(PPh3)}2M]ClO4 (M = Ag, Au), CNC = 2,6‐diphenylpyridinate. The complexes act as models for intermediates in transmetalation processes.
The X‐ray crystal structures of some of the complexes confirmed the existence of Pt−M bonds. NMR spectra showed evidence for a dynamic behavior in which the Au/Ag–C bonds are broken and reformed in solution. The interaction between Ag or Au and the carbon atom of one of the phenylene rings in these heteropolymetallic complexes can be considered as the first step in a process of interchange of aryl ligands. Due to the nature of the ligands, the process is frozen at this step in the model complexes. The data collected sheds light on the properties and behavior of the Pt–Au/Ag bimetallic systems.
- Pt–M Complexes (M = Ag, Au) as Models for Intermediates in Transmetalation Processes,
Miguel Baya, Úrsula Belío, David Campillo, Israel Fernández, Sara Fuertes, Antonio Martín,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201802542