Heteroybiaryls consisting of a phenol and a benzo[b]thiophene moiety are useful scaffolds for pharmaceutical ingredients and precursors for high-performance electronic devices. However, the currently available syntheses of these compounds require the use of many reagents or harsh reaction conditions and often result in large amounts of waste. Therefore, a direct and more sustainable route to such molecules is highly desirable.
Siegfried R. Waldvogel, University of Mainz, Germany, and colleagues have developed a direct electrochemical protocol allowing a highly regioselective cross-coupling of phenols with benzo[b]thiophenes. When applying an electric current as a reagent, no leaving and protecting groups are required, and no toxic and precious metals are required as catalysts. The setup is simple, using only a beaker with two electrodes.
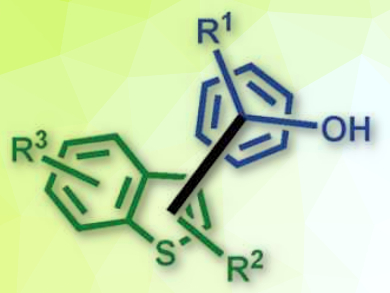
The key to the successful electrochemical coupling is the use of a stabilizing media such as 1,1,1,3,3,3‐hexafluoroisopropanol (HFIP). It is capable of stabilizing reactive intermediates generated at the anode, while being stable under the same electrolytic conditions. The developed electrochemical protocol enables the selective access to the desired products (pictured) in high yields. A broad scope of functional groups is tolerated and the only by-product is hydrogen.
- Regioselective Metal- and Reagent-Free Arylation of Benzothiophenes by Dehydrogenative Electrosynthesis,
Sebastian Lips, Dieter Schollmeyer, Robert Franke, Siegfried R. Waldvogel,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201808555



![Calix[4]arene “Handshakes” via Urea–Carboxylate interactions](https://www.chemistryviews.org/wp-content/uploads/2024/04/calixarenehandshake_2024-125x94.png)