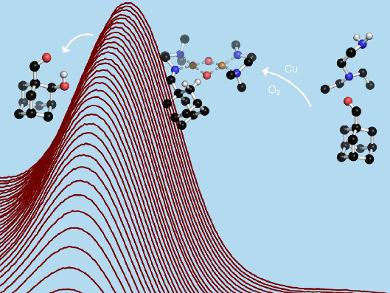
Despite great industrial demand, the use of molecular oxygen from air as an oxidant is often problematic. Nature uses iron or copper enzymes to perform such reactions under ambient conditions. The development of synthetic compounds that mimic this activity has been a long-standing research goal.
Andrey A. Fokin, Igor Sikorsky Kiev Polytechnic Institute, Ukraine, and University of Gießen, Germany, Max C. Holthausen, University of Frankfurt am Main, Germany, Siegfried Schindler, University of Gießen, and colleagues have developed a simple “clip-and-cleave” concept for selective oxidation reactions. The team achieved efficient hydroxylations of unactivated aliphatic β-C–H bonds with high selectivity.
The synthesis involves the reaction of an aldehyde with N,N‐diethylethylenediamine to give the corresponding imine, which is used as a bidentate donor ligand forming a copper(I) complex with [Cu(CH3CN)4][CF3SO3]. The complex then reacts with dioxygen (“clip”), and as a final step, an acidic cleavage (“cleave”) gives the corresponding β‐hydroxylated aldehyde.
This approach provides access to 1,2-disubstituted adamantane and diamantane derivatives that are otherwise difficult to prepare. As an extension of the concept, the amine could be immobilized, for example, on a silica matrix, which should allow the hydroxylation of aldehydes under mild conditions using only dioxygen from air.
- Aerobic Aliphatic Hydroxylation Reactions by Copper Complexes: A Simple Clip-and-Cleave Concept,
Jonathan Becker, Yevheniia Y. Zhyhadlo, Ekaterina D. Butova, Andrey A. Fokin, Peter R. Schreiner, Moritz Förster, Max C. Holthausen, Pascal Specht, Siegfried Schindler,
Chem. Eur. J. 2018.
https://doi.org/10.1002/chem.201802607