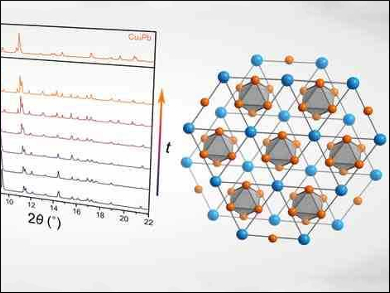
Exploring uncharted areas of phase space enables the discovery of exotic materials. One area of particular interest is the combination of transition metals with heavy main-group elements. One approach to accessing such materials is the use of high pressures.
Danna E. Freedman, Northwestern University, Evanston, IL, USA, and colleagues have used high-pressure synthesis to prepare the first binary compound of copper and lead, Cu3Pb. The team created Cu3Pb by subjecting a mixture of copper and lead to pressures of 16 GPa (over 100,000 atm), while simultaneously laser-heating the sample to temperatures greater than 1000 K. The pressures were achieved by compressing the metals inside a diamond anvil cell. The reaction was monitored using in-situ powder X-ray diffraction.
Cu3Pb can be best described as a mixture of the two elemental structures, with a hexagonal lead lattice hosting octahedra of copper atoms (pictured). The high formation pressure suggests the hexagonal high-pressure structure of elemental lead is a prerequisite for the formation of Cu3Pb. Electronic structure calculations indicate that Cu3Pb may show an unusual electronic structure, which is sensitive to small structural changes. This means chemical doping could potentially lead to the isolation of doped Cu3Pb at ambient conditions.
- Discovery of Cu3Pb,
Alexandra D. Tamerius, Samantha M. Clarke, Mingqiang Gu, James P. S. Walsh, Marco Esters, Yue Meng, Christopher H. Hendon, James M. Rondinelli, Steven D. Jacobsen, Danna E. Freedman,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201807934