Carbon monoxide is a toxic, but highly useful gas. It is used to produce basic chemicals such as hydrogen or aliphatic aldehydes and acids. The carbonylation of aliphatic compounds involves two electrons transferred on carbon monoxide—and no more. The carbon–oxygen bond seems to be too strong to strip the molecule bare of its oxygen in a single catalytic step.
Deborah L. Kays, University of Nottingham, UK, and colleagues have reported such a transformation for the first time. Under a carbon monoxide atmosphere, low-coordinate iron complexes reduced and cyclized carbon monoxide into a four-membered ring, the core of an unusual squaraine molecule. Four electrons were transferred in this reduction.
Low-Coordinate Iron Complexes
Many metals can coordinate carbon monoxide and activate it to form carbon–carbon bonds. This reaction is used in the Monsanto and Reppe carbonylation processes, where carbon monoxide adds to C1 or C2 hydrocarbons to produce, e.g., acetic acid, propanoic acid, or acrylic acid, catalyzed by metal carbonyl complexes.
Here, the number of electrons transferred onto carbon is never more than two, and the carbon retains its oxygen. Stripping carbon monoxide bare of its oxygen would require six-electron reductions. The researchers were able to demonstrate such a reduction and carbon-carbon bond formation. They investigated low-coordinate iron complexes, i.e., complexes where the number of coordinating ligands is smaller than usual, for their reducing potential. In iron terphenyl complexes, iron(II) binds to the central phenyl ring of three connected phenyl rings, and in the diterphenyl complex investigated by the team (one iron binds two terphenyls), the central iron is deeply buried.
Squaring Carbon Monoxide
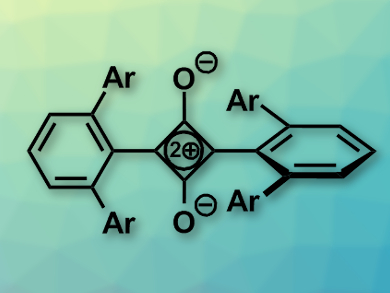
When stirring this iron diterphenyl complex under a carbon monoxide atmosphere in toluene solution, the yellow solution turned dark red, the researchers reported. They isolated and characterized the red crystals and found a surprising squaraine-type structure (pictured). Squaraines are highly fluorescent dyes containing the four-membered carbon ring of squaric acid in its center, but with two oxygen atoms of squaric acid replaced by aromatic entities. The structure is planar and fully conjugated.
The squaraine molecule isolated by the team was different—it was not planar. Its core was the unsaturated four-membered squaric acid ring, but the two terphenyls flanking the ring were twisted, which was not surprising regarding the steric demand of the bulky ligands. The scientists characterized their squaraine as having “atypical electronic and bonding properties,” which result in “broken conjugation.”
Labeling studies showed that the squaraine inner four-carbon ring was indeed made of four carbon monoxide molecules. This means that at least two carbon monoxide molecules in this reaction were entirely stripped of their oxygens. Another reaction product was a diiron tetracarboxylate complex. A third product was iron(0) pentacarbonyl. Balancing the reaction, the scientists concluded that two iron(II) atoms were reduced by two electrons each, CO was net reduced by four electrons, and eight terphenyl ligands were oxidized by a total of eight electrons. Carbon–carbon bonds in the products replaced the educt carbon–iron bonds.
Carbene and Ketene Intermediates
The scientists proposed several insertion and migration steps of carbon monoxide, with interesting carbene and ketene intermediates, in this reaction. Although carbon monoxide coordination and migration are common phenomena in transition metal complexes, this low-coordinate iron complex reduced carbon monoxide further than usual. The strong C–O bond in carbon monoxide seems to have its weak point.
The team emphasized the ambient conditions under which this transformation proceeded. More unconventional and exciting mechanisms for seemingly “old” reactions may be discovered.
- Selective reduction and homologation of carbon monoxide by organometallic iron complexes,
Helen R. Sharpe, Ana M. Geer, Laurence J. Taylor, Benjamin M. Gridley, Toby J. Blundell, Alexander J. Blake, E. Stephen Davies, William Lewis, Jonathan McMaster, David Robinson, Deborah L. Kays,
Nat. Commun. 2018.
https://doi.org/10.1038/s41467-018-06242-w