Anthrax disease is caused by a bacterium called Bacillus anthracis, which produces hazardous toxins. To acquire essential iron from its host, B. anthracis excretes two siderophores that bind Fe(III) ions—bacillibactin (BB) and petrobactin (PB). Human siderocalin in plasma binds and neutralizes BB, however, no natural scavenger is available for PB.
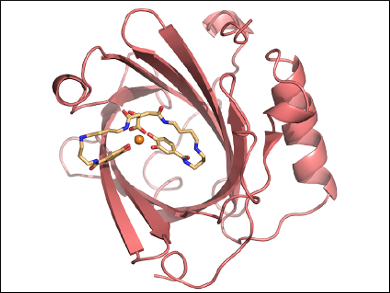
Arne Skerra and colleagues, Technical University of Munich, Germany, have reshaped the ligand pocket of siderocalin to tightly bind Fe(III)-PB instead of Fe(III)-BB. Combinatorial protein design was used to prepare a mutant lipocalin. This “petrocalin” (pictured) carries a total of 24 amino acid exchanges around the ligand pocket and complexes Fe(III)-PB with an extremely low ~20 pM dissociation constant.
Microbiological studies with a related Bacillus cereus strain that excretes BB and PB under conditions like that in the human body, but does not produce hazardous toxins, revealed that a combination of petrocalin and siderocalin strongly inhibits bacterial growth in culture medium. The researchers believe that therapeutic treatment of life-threatening anthrax infections may be possible by administering petrocalin to act in combination with the natural human siderocalin in the body.
- Reprogramming human siderocalin to neutralize petrobactin, the essential iron scavenger of Anthrax bacillus,
Martin Dauner, Andreas Eichinger, Genia Lücking, Siegfried Scherer, Arne Skerra,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201807442