Oligonucleotide therapeutics, such as antisense oligonucleotides (ASOs) or short interfering RNA (siRNA), can modulate gene expression. It is possible to restore or delete gene function, as well as up- or down-regulate protein expression.
In drug discovery, stable isotope-labeled compounds are used for multiple purposes, specifically in mass spectrometric techniques. Molecules with a different molecular weight that maintain identical chemical, biological, and physical properties allows for precise quantification and imaging in various assays.
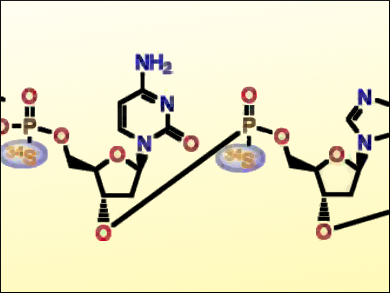
Anders Dahlén, AstraZeneca, Mölndal, Sweden, and colleagues have developed a convenient, fast, and general method for 34S stable-isotope-labeling of oligonucleotides with a phosphorothioate backbone (pictured). The method is optimized for automated oligonucleotide synthesis. The team first developed a highly efficient, two‐step, one‐pot synthesis of 34S‐labeled phenylacetyl disulfide (34S‐PADS). The synthesis starts from 34S‐enriched elemental sulfur, followed by a reduction with Super‐Hydride and a treatment of the resulting Li2S2 species with phenylacetyl chloride. 34S‐PADS was then used for the labeling of oligonucleotides.
The 34S‐labeled oligonucleotides were shown to have the same melting temperature, activity, and secondary structure as those of the corresponding unlabeled 32S-oligonucleotide. 34S-labeled oligonucleotides could be useful as internal standards for quantification in biological matrices, for the exploration of biotransformations, for metabolite studies, for biodistribution studies, etc.
- A Versatile and Convenient Synthesis of 34S-Labeled Phosphorothioate Oligonucleotides,
Rouven Stulz, Johan Meuller, Dženita Baždarević, Charlotte Wennberg Huldt, Roger Strömberg, Shalini Andersson, Anders Dahlén,
ChemBioChem 2018.
https://doi.org/10.1002/cbic.201800417