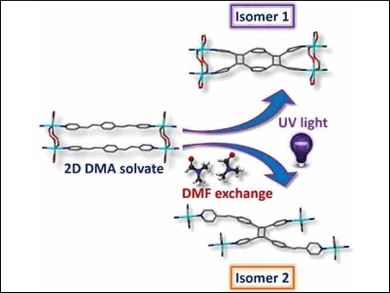
Metal–organic frameworks (MOFs) and porous coordination polymers (PCPs) have been used to facilitate [2+2] cycloaddition reactions in the solid state. However, so far, controlling which isomer of the product is formed has required the preparation of distinct precursor materials.
Leonard Barbour and colleagues, University of Stellenbosch, South Africa, have synthesized a Cd(II)-based PCP that enables the synthesis of either of two cyclobutane isomers in the same PCP. Control over the product is achieved by simple exchange of the solvent guest within the pores.
The team carried out the photochemical [2+2] cycloaddition of 1,4-bis[2-(4-pyridyl)ethenyl]-benzene in the PCP using UV light, first with dimethylacetamide (DMA) as a solvent guest in the coordination polymer to obtain one specific product isomer. When the PCP was soaked in dimethylformamide (DMF) before irradiation, a different isomer of the product was obtained (pictured).
Single-crystal X-ray diffraction techniques showed that the solvent induces subtle changes in the conformation of the reactands, which then dramatically alters the outcome of the photochemical reaction. The products were successfully extracted from the PCP and characterized spectroscopically. This work demonstrates a new way of achieving control over the outcome of photochemical reactions in the solid state.
- Solvent-mediated synthesis of cyclobutane isomers in a photoactive cadmium(II) porous coordination polymer,
Leonard Barbour, Isabella Claassens, Varvara Nikolayenko, Delia Haynes,
Angew. Chem. Int. Ed. 2018.
https://doi.org/10.1002/anie.201809050